What Is Wrong With VAERS/FAERS
Let me count the ways...
Dear FDA / Dr. Tracy Beth Hoeg,
It is the opinion of this author and creator of vaersaware.com after five years of almost exclusive pharmacovigilance systems auditing (VAERS, FAERS, VSAFE, EMA, MEDSAFE) that very little data published is organic. Moreover from my expert HMO Claims Auditing and revenue cycle management experience, it’s is my belief data is being manipulated and obfuscated during the adjudication process and before initial publication. In addition to this data malfeasance, I’ve come to the conclusion that VAERS at minimum does not even publish all legitimate reports received, as voiced by your boss Robert F. Kennedy Jr. HERE. My "General Dynamics” series of articles HERE. If in fact VAERS does not even publish all legitimate reports received it would render the rest of this message moot, however I will describe the data obfuscations of the reports that have managed to be made public which is also summarized in PowerPoint and bullet point fashion HERE.
The over arching umbrella of obfuscations is the adjudication and publication of initial reports. On the one hand per CDC guidelines there is a very reasonable four to six week adjudication phase to determine reports are authentic and not fake, false or duplicate. It’s assumed during this process adjudicators request additional information from submitters where applicable to publish the most complete VAERS report possible. This includes very basic but critical data like Age, Gender, State Location, Vax, Onset and Death Date, Adverse Event Level, and a abbreviated summary narrative that healthcare professionals might understand as a SOAP note (Subject, Object, Assessment, Plan). Other critical data elements like previous vax history, lab data, Other Medications and Current Illness where applicable would be an absolute dream to see published, but these fields are so scarcely published that it is a problem unto itself.
Lastly, the lack of actual vaccine(s) information published like Vax Type, manufacture name, Lot number, dose series, and vaccine name is actually appalling if not malfeasance or willful misconduct? It does not need to be stated to you FDA and Dr. Hoeg, but rather for this audience that ~85% of all reports submitted/published are submitted by some healthcare professional, pharmacy, hospital, county health department, or vaccine manufacturer as opposed to some non-medical professional or family member.
I will assume every VAERS adjudicator is also a Certified Professional Coder (CPC) capable of extracting information from anywhere in the report to determine what MedDRA codes will be applied. In the professional world every claims adjudicator is also a CPC and only the best adjudicators would become auditors, and the best auditors become Lead Auditors like myself. I don’t know how the VAERS department segregates job functions and responsibilities but there is straight up fraudulent data manipulation and obfuscations going on within the department in my opinion. FAERS is no better but I’ll get to them later.
FDA and Dr. Hoeg,
Please consider this my appeal and grievance FDA/CDC but not from vaersaware.com or the general public but on behalf of my now disabled uncle whom I filed his VAERS report and what ever authority that grants me. See ID# 1342095
In adjudicating my report submission CDC/FDA created symptoms (medDRA) he simply did not have, you deleted phrases and wording from my sacred first hand summary narrative, you in effect changed the story I was trying to convey. It’s one thing to delete hospital names or attending physician names or any patient identifying information under HIPAA, but that’s not what you did. By deleting phrases and wording you change my first hand account and what I was trying to convey! Luckily I video captured my live submission back in May 2021 and made a tree part series starting HERE
FDA needs to employ the same CMS auditing process style known as ODAG. An ODAG report refers to documentation and data outputs generated as part of the Organization Determinations, Appeals, and Grievances (ODAG) audit process conducted by the Centers for Medicare & Medicaid Services (CMS). Key Details:
Purpose: ODAG audits evaluate an HMO’s compliance with federal regulations for handling member requests related to coverage determinations (e.g., approvals/denials of services or payments), appeals of those decisions, and grievances (complaints about quality of care or service). This includes assessing timeliness, clinical appropriateness, effectuation of decisions, and processing requirements. It’s a critical component of broader CMS program audits to ensure HMOs deliver services correctly and avoid improper payments or denials.
What the Report Includes: The “report” typically encompasses:
Universe Tables: Structured data extracts (e.g., Tables 1–5 in CMS protocols) listing all relevant cases over a specified period (often 1–2 years). These include details like request dates, decision types (e.g., payment vs. non-payment), authorization/claim numbers, notice dates, and outcomes.
Audit Findings: Compliance scores, error rates, and recommendations based on sampled cases from the universe. For example, CMS tests for standards like processing payment requests within 60 days or providing timely notices.
Supporting Protocols: Guidance on data requests, evaluation methods, and enforcement actions if deficiencies are found (e.g., corrective action plans for HMOs).
Process Overview:
CMS issues an ODAG Audit Process and Universe Request to the HMO, requiring submission of raw data in specified formats (e.g., CSV with fields like receipt dates and decision rationales).
A sample of cases (e.g., 95–400 per category) is reviewed for compliance.
Results are compiled into an enforcement report, which may lead to financial recoveries, monitoring, or sanctions if error rates exceed thresholds (e.g., >10% in timeliness).
Relevance to Claims Auditing: In HMO claims workflows, ODAG reports help auditors (internal or CMS) identify overpayments, underpayments, or procedural errors in claims processing. They’re distinct from coverage determinations audits (CDAG) but often overlap, as both tie into claims validation. HMOs use these reports for internal quality improvement, training, and risk management to prepare for annual CMS audits.
This framework is outlined in CMS’s official Program Audit Protocol (e.g., Form CMS-10717) and annual enforcement reports, which emphasize transparency and corrective actions to improve beneficiary access and reduce audit failures. If you’re referring to a specific HMO or year, more tailored details could be available via CMS’s audit portal.
VAERS Data Obfuscations:
Unknown Age: Ridiculous if not fraudulent that 30% of all Covid-19 reports which is 8% above a already horrible 22% for all other vax types. This is a great example of the hidden 400 dead kids that received a Covid-19 vaccines HERE.
Unknown State Location: 31% of all Covid-19 domestic death reports. 16.5% unk state for all other Covid-19 reports (except death). How is it possible the most severe and most vetted and scrutinized SAE has double the unknown states? Fraud, that’s how.
Mass Deletion of Reports: Over 32,000 Covid-19 have been deleted after publication, and over 1,300 of which are death reports. A technique of “de-duplicate” conditional matching against live reports many legitimate reports seem to have been deleted in error or fraudulently. Please contact me for further details. Moreover, legitimate deleted reports should be recognized and identified as fake, false, duplicate and still be published yet segregated from true live population of reports. This is how we do it at vaersaware.com
Throttling (Purposeful delay of publication): ~20% of all 1.6M Covid-19 were initially published outside the six weeks max time limit adjudication window. Of which ~58K reports were published four months after received. Hundreds of the most extremely throttled death reports one, two, and three years after publication, see HERE.
Under Coding Adverse Events: 100K serious adverse events are coded as “not serious” even some uncounted deaths. MedDRAs like cardiac arrest, stillbirths, myocardial infarctions, pulmonary embolism, etc. are coded as not serious and even below an office visit aka None of Above.
Bundling Multiple Deaths: At least 2,500 worth of bundled C19 deaths are tied up over hundreds of reports. Bundling multiple patients, registrants, or victims into a single report is not exclusive to just death reports. However there could easily be tens of thousands of extra reports if theoretically unbundled.
Down Coding Severe Events: Kind of self explanatory, but sometime after initial publication a death can be down coded to the reports next highest event level. Some times a report can get down coded for serious to non-serious by definition. A disclaimer here is in a few rare cases a report has been up coded to even death. However some of these deaths that have been up coded after initial publication happened as much as two years after initial publication.
Manufacture & Lot Mismatches: Thousands of reports and line items have a Pfizer name but Moderna Lot number and vice versa and every combination including Janssen.
Lot Number Typos: Self explanatory but to generalize about 60,000 unique C19 lot numbers could be cut down to about 30,000 unique lot numbers just by ethically fixing typos and special characters. Z to 2, O to 0, I to 1, etc. etc. etc.
Miscoding Covid Vax Type as Unknown: Unknown Vax Type has exploded ever since the C19 rollout.
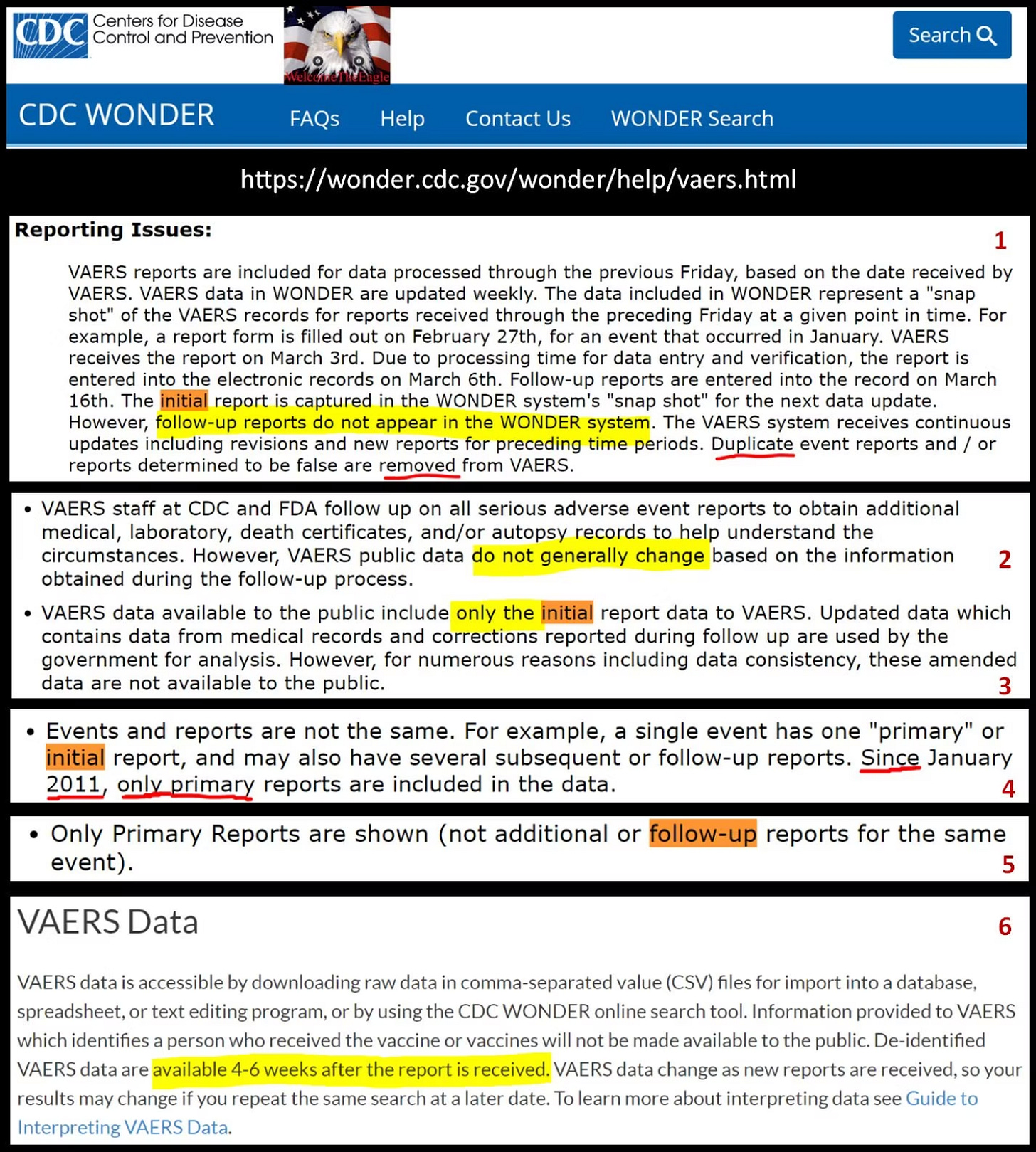
Publishing ONLY Initial Reports: From 1990 to 2010 initial published reports were being updated with follow-up data. Not until after the Harvard Pilgrim Study was a paradigm shift made to publish ONLY initial reports (see blurb #4).:
Publishing Reports To Quickly: Finalizing and publishing reports within 24hrs-72hrs only to have them deleted in the next cycle presents it’s own set of problems. There is a general problem of incomplete data regardless of to soon or very late time lags. However generally speaking the supposed heavily scrutinized are are being finalized entirely too fast and with mountains of missing data.
Radical Transparency now the publication of follow-up reports if a farce so far:
This expanded public access and now publishing follow-up reports has been for all intent a purposes a failure so far especially for Covid-19 reports and really for all vax types with a received of about 2010 or ~2015. Generally speaking I agree with medalerts.org that only about ~64,000 reports have been affected, very few of those have been Covid-19 reports or about ~4,000 Covid-19 reports now have follow-up data. However, there is now and additional ~950 now since dead but alive during initial submission. It must be stated that some victims even died in a follow-up report but during the initial adjudication phase. Meaning VAERS could have up-coded to dead even during initial adjudication but did not. If this is not obfuscation I don’t know what is.
Arbitrary 20min Time Limit To File: The easiest fix would probably make the biggest impact. I have personally heard this same story often regular people getting timed-out and frustrated and quitting and one or multiple attempts. This max time limit is totally arbitrary and should be extended by 10min, 15min, or even one hour would be a humongous help. If really compassionate VAERS would allow a “save” feature to come back later and finish the report.
Lack of Interactive Public Dashboard: Self explanatory except to say, not a trimmed down pathetic Qlick example that FAERS uses. FAERS dashboard is an insult to Qlick and another example that we are not dealing with genuine honesty and radical transparency like I keep hearing. Please see my previous articles on FAERS HERE.
Supervised or Unsupervised Data Extraction: Summary narratives contain a wealth of information and is the primary source where VAERS CPC will do their coding from to assign MedDRA to reports. VAERS needs to do a better job at dynamically and manually verify performance. Many coding error are being made and therefore proper MedDRAs are not being assigned, additional lot numbers go unrecognized, and generally speaking all forms of typo are allowed to passthrough into publication unabated. Ethical “data cleansing” needs to be done and any of these ethical correction need to be allowed into publication.
Conclusion:
I know the solutions to all these problems a really minor to moderate in nature and nothing new under the sun. I see the processing of these reports are 95% similar to how insurance claims are processed in the HMO and PPO insurance world. Internally I imagine the health records software modules being used is very similar if not exactly similar to anything out in the market today as in EPIC, GE Centricity, AdvancedMD, etc. etc. etc.
To be clear I am under no illusion that very much of what the public is currently allowed to see is organic in nature. I do not believe that all missing data is organic and that VAERS simply did not request additional info during the adjudication phase, but maybe obtained additional requested data sometime after initial publication? My real expert assertion is that something nefarious is going on and VAERS is even dynamically scrubbing critical data that was there on initial reports and are obligated to publish but simply fraudulently do not. They proved how easily and dynamically this can be done by currently scrubbing all the EMA and MHRA foreign data to this very day.
AI and faster processing times will never be a substitute for honest accountability. AI without honest pharmacovigilance and radical transparency will have the opposite effect. CDC/FDA/HHS will just get better at fraud without the integrity part. A radical idea would be to also bring all the V-Safe data into VAERS. An absolute horrible idea would be to move VAERS into FAERS in huge part because raw data downloads be effectively impossible. The only “plus” FAERS has it’s concomitant drugs organizational feature, but currently lacks any summary narrative section which would be a huge lose disadvantage. FAERS also doesn’t have dedicated vax date, or onset date or death date fields. The whole FAERS dashboard and database basically sucks and it not meant for any meaning public inspection or analysis. It does have over 30M records. As an auditor I feel like a gorilla at the zoom who can’t get to the banana on the other side of the window. God Bless
Lastly and with reiteration, I think I properly and reasonably corroborate what Robert F. Kennedy said at my church on June 19, 2021, he was told by VAERS adjudicators, 150K reports had disappeared from their queues (not published).









in Italia ognuno può fare denuncia di effetto avverso a VigiFarmaco, ma SOLAMENTE SE LORO RITENGONO i sia CAUSALITà allora viene pubblicato nel sito e poi inviato, come di prassi all'EMA.
la denuncia che ho fatto per mia mamma NON C'é PIù.
ho denunciato anche su VAERS. la enuncia è ancora presente.
My simple fix is this. Every report submitted is assigned a case manager. Said case manager contacts victim/reporter and reviews all facts, missing fields, other doses given that visit
etc. And victim is given the VAERS ID and any pertinent information on next steps if any. Simple. Thank you for representing me - a victim in VAERS and a reporter of Mom's death report.